( Current upated information as of 25-Mar-2021 )
By Farheen Khan, (Hons)B.Sc. (Biochemistry) [Get Well Clinic]
It is now the month of March, and a few more vaccine candidates have marched into our COVID-19 vaccine toolkit. These include the newly approved Oxford-AstraZeneca and Janssen-Johnson & Johnson vaccines.
Both vaccines are viral vector vaccines.1 More specifically, these vaccines contain a weakened, live adenovirus - a virus that causes the common cold.2 To develop these vaccines, scientists first removed all disease-causing and replication-related genes from the adenovirus.1,3–5 As the adenovirus can now no longer replicate or cause disease, it is harmless to the human body.1,3–5
Next, scientists inserted the DNA, or genetic instructions, for the SARS-CoV-2 spike protein into the modified adenovirus.1
Now, you may be wondering, “why the spike protein?” This is because it is the spike protein of the SARS-CoV-2 virus that interacts with a specific receptor, the ACE-2 receptor, on the surface of our cells.6 Upon interaction, the virus is internalized into our cells, and proceeds to take over our cells’ machinery, repurposing them to create more copies of the virus. These infected cells then release millions of copies of the virus which then go on to infect other cells in our bodies, ultimately causing the COVID-19 disease.7
Once injected with the Oxford-AstraZeneca or Janssen-Johnson & Johnson vaccine, the adenovirus acts as a “viral vector,” or tool, to deliver the DNA of the SARS-CoV-2 spike proteins to the cells of the vaccinated individual.1,3–5 The adenovirus enters the cells, and inserts their genetic information, along with the spike protein DNA, into the vaccinated individual’s cells’ nuclei.1,3 Our cells’ machinery then transcribes this DNA into RNA in the nucleus of our cells.8 This RNA is then processed to become mature messenger RNA (mRNA).8 After this, the mRNA is transported to the cytoplasm of our cells (thus called messenger RNA), and is decoded and translated into the spike proteins, which are then presented on the surface of the vaccinated individual’s cells.8
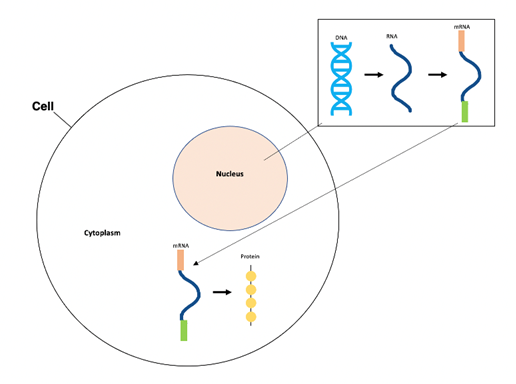
Figure 1. The central dogma process includes DNA transcription into RNA (in nucleus), RNA processing to mRNA (in nucleus), and mRNA translation to protein product (in cytoplasm).
The presence of the spike proteins, and the adenovirus itself, then elicits an immune response as the individual’s body recognizes the protein and adenovirus as foreign substances.1–5 More specifically, the immune system produces antibodies against the protein and adenovirus, and executes other necessary immunogenic responses as well.9–11 Since only one protein of the virus would be created, rather than the whole virus itself, the vaccine cannot infect a vaccinated individual with COVID-19.1
As a note, antibodies are large Y-shaped proteins that stick to the surface or part of a virus or bacteria, tagging it for attack by other elements of the immune system to ultimately neutralize the virus or bacteria.12 Antibodies are designed to bind to and attack one kind of virus or bacteria only.12 This means that an antibody that is for instance designed to destroy the Influenza A virus, cannot be used to attack the SARS-CoV-2 or EBOV viruses.
As antibodies specific to the SARS-CoV-2 spike protein are produced upon administration of the Oxford-AstraZeneca or Janssen-Johnson & Johnson vaccine, if the SARS-CoV-2 virus does enter the vaccinated individual’s body at a later time, the antibodies produced post-vaccination will be able to recognize the spike proteins of the virus and thus bind to them.4,5,10,13 As a result, the virus will be unable to bind to the ACE-2 receptors on the surface of the individual’s cells, and therefore will not be internalized, rendering it incapable of taking over the cells’ machinery and replicating.10,13 In addition, the binding of the antibodies to the spike proteins present on the surface of the SARS-CoV-2 virus will also tag the virus for degradation by other elements of the immune system.10,13
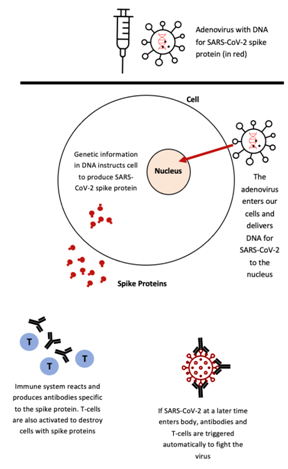
Figure 2. The engineered adenoviral-vector containing the DNA for the SARS-CoV-2 spike protein is injected into an individual’s body. The DNA is transcribed into mRNA, which is then translated into a fragment or version of the SARS-CoV-2 spike protein in the cytoplasm of the individual’s cells. As the spikes are released from the individual’s cells, their immune system produces antibodies and releases T-cells to execute necessary immunogenic reactions. This image has been adapted from a Figure in The Washington Post’s “Your questions about coronavirus vaccines, answered” under “How does the Johnson & Johnson vaccine work?”.13
It is important to note that the adenovirus cannot replicate itself and does not have the necessary machinery to integrate into their hosts’ DNA. As such, an individual’s DNA will not be altered upon vaccination.1,3,11 Further, viral-vector vaccines tend to be less effective if the viral-vector in use is a virus that people may already have antibodies for.3,11 If this occurs, then the antibodies would overwhelm the adenovirus before it even gets the chance to deliver the spike protein DNA.3,11 As such, scientists at Oxford-AstraZeneca and Janssen-Johnson & Johnson have ensured to use adenoviruses that humans are unlikely to have encountered before.4,5 More specifically, the Oxford-AstraZeneca vaccine uses a modified version of a chimpanzee adenovirus, and the Janssen-Johnson & Johnson vaccine uses a recombinant, or modified, human adenovirus (adv26) - the same adenovirus that has been used in the company’s Ebola virus vaccine.4,5
Now that we know how these viral-vector vaccines work, I would like to mention that this technology, unlike that of the mRNA vaccines, is not new.3,11 Research regarding adenoviral-vector vaccines date back to the 1990s.11 Adenoviruses were originally studied to be used in gene transfer therapy for diseases including cystic fibrosis.11,14 Though they seemed promising at first, it unfortunately did not work out as adenoviruses induce strong immune responses, causing them to be removed by our immune system fairly quickly, thus limiting their purpose in gene therapy.11,14 However, this very characteristic is what makes adenoviruses excellent candidates for vaccine development against infectious diseases.1,3,11 As such, scientists have worked on adenoviral-vector vaccines against a multitude of diseases including HIV, Zika and Tuberculosis.5,11 Further, adenoviral-vector vaccines were produced and distributed to West Africa and the Democratic Republic of the Congo during the Ebola outbreaks in the past decade.5,11,15
Aside from the fact that these vaccines can induce strong immune responses and that they are a well-established technology, the Oxford-AstraZeneca and Janssen-Johnson & Johnson vaccines bring additional advantages over the Pfizer-BioNTech and Moderna vaccines.3 For instance, both adenoviral-vector vaccines can be stored in normal 2°C-8°C refrigerators for up to three to six months, making distribution of these vaccines easier.16,17 The Janssen-Johnson & Johnson vaccine also only requires one shot, while the others require two shots - something that can be of great help if we fall low in vaccination supply.17,18 For more comparisons between the Pfizer-BioNTech, Moderna, Oxford-AstraZeneca, and Janssen-Johnson & Johnson vaccines, please refer to Table 1 below.
So, which vaccine is the best vaccine to take? They all are. Despite their differing overall efficacies, all vaccines have been approved for use in Canada, and are all 90-100% effective in preventing the worst outcomes: hospitalizations and death.19 It’s important that you get vaccinated with whichever vaccine is available to you as soon as possible to prevent the worst possible outcomes from COVID-19 infection. It is also important to remember that for the vaccination to be effective, we must combine the immunization strategy with continuous mask-wearing, hand-washing, and social distancing. We are multiple steps closer to potentially winning the battle against this life-changing virus than we were at the start of this year. We need to continue to remain cautious as everyone gets vaccinated so that we can ensure our success, and hopefully go back to the pre-COVID-19 times, when lockdowns, stay-at-home orders, and indoor capacity restrictions were not parts of our daily lives.
DISCLAIMER: You may have heard on the news that several countries in the European Union suspended administration of the Oxford-AstraZeneca vaccine due to safety concerns.20–22 Out of approximately 20 million European residents who received their first dose of the Oxford-AstraZeneca vaccine, 30 individuals were reported to have developed blood clots.20 After conducting a review of the safety data, the European Union’s medicine regulator has since deemed the vaccine to be safe, and has even said that the benefits of the vaccine outweigh the risk of side effects.20,23 To put this into perspective, in the United States, 1 to 2 adults per 1000 are affected by venous thromboembolism (blood clots in the veins) each year, with higher risk in men, and individuals of older age.24,25 In fact, the World Health Organization even states that “venous thromboembolism is the third most common cardiovascular disease globally.”26 Furthermore, the incidence of venous thromboembolism associated with the COVID-19 disease is 21%, which further increases to 31% in ICU-related cases.27 As such, by vaccinating individuals, and thus reducing their risk of severe COVID-19 disease and complications, the overall risk of developing blood clots would actually be reduced. Thrombosis Canada also states, “there is no link between receiving this vaccine and the development of blood clots … vaccines of any type are not associated with the development of blood clots.”22 Finally, according to an interim analysis report regarding the U.S. Phase 3 trials published on March 22, 2021, “the data safety monitoring board conducted a specific review of thrombotic events, as well as cerebral venous sinus thrombosis (CVST) with the assistance of an independent neurologist … (and) found no increased risk of thrombosis or events characterised by thrombosis among the 21,583 participants receiving at least one dose of the vaccine.”28
TABLE 1. Table to Summarize the Similarities and Differences between the Two Vaccines*
|
|
Pfizer-BioNTech Vaccine |
Moderna |
Oxford-AstraZeneca |
Janssen-Johnson & Johnson |
|
Type of Vaccine |
mRNA29 |
mRNA29 |
Non-replicating adenoviral vector30 |
Non-replicating adenoviral vector17,31 |
|
Shelf-life at Normal Refrigeration Temperatures (2°C-8°C) |
5 days29 |
30 days29 |
6 months30 |
3 months17 |
|
Dosage Required |
Two 0.3mL doses (30μg of mRNA each); 21 days apart29 |
Two 0.5mL doses (100μg of mRNA each); 28 days apart29 |
Two 0.5mL doses (5x1010 viral particles each); 12 weeks apart30,32
|
One 0.5mL dose (5x 1010 viral particles each)5 |
|
# of Phase 3 Trial Participants |
> 43,00029 |
> 30,00029 |
> 17,00030 |
> 43,00017 |
|
Participants’ Age and Health Conditions |
Age groups: 12-15 (100 participants only); 18-55; 65-85 Health Conditions: Healthy individuals with and without prior SARS-CoV-2 infection; Individuals with stable pre-existing disease; Individuals with stable, chronic HIV, HCV, and HBV infection29 |
Age: 18+; Health Conditions: Healthy with no previous history of SARS-CoV-2 infection; Individuals with stable pre-existing medical conditions29 |
Age: 18-65; Health Conditions: Healthy individuals; Individuals with stable pre-existing medical conditions33 |
Age: 18+; Health Conditions: “Adults with and without comorbidities (most common comorbidities were obesity and hypertension) associated with increased risk of progression to severe COVID-19”5 |
|
Trial Design |
Randomized, blinded, placebo-controlled29 |
Randomized, blinded, 1:1 placebo-controlled29 |
Randomized, blinded, placebo-controlled30 |
Randomized, blinded, placebo-controlled5,17 |
|
Placebo Used |
Saline solution29 |
Saline solution29 |
Meningococcal vaccine or saline solution33 |
Saline solution5 |
|
Overall Efficacy |
95% (>94% in individuals over the age of 65)29 |
94.1%29 |
54.9%-82.4%34** |
66%17 |
|
When was Vaccine Efficacy Measured? |
7 days after second dose (day 28)29 |
14 days after second dose (day 42) 29 |
At least 15 days after second dose (day varied) 34 |
14 days and 28 days after initial dose (day 14, 28)17 |
|
Observed Side Effects |
Fatigue, Headache, Pain at Injection Site29 |
Fatigue, Muscle Aches, Headache, Pain, Redness at Injection Site29 |
Fatigue, Headache, Pain and Tenderness at Injection Site, Feverishness, Muscle Aches35 |
Fatigue, Headache, Pain at Injection Site, Muscle Aches36 |
|
How Long Will Participants Be Monitored? |
2 years after second dose29 |
2 years after second dose29 |
2 years after second dose in U.S. trials37 |
2 years after vaccination5,17 |
*Note: These are initial clinical study trial data available by December 2020 in trials of less than 100,000 people, and short-term endpoints. The real-world results that are coming out in 2021 as over 100 million people are being vaccinated with COVID-19 vaccines likely will show even better results.
**Oxford-AstraZeneca conducted four randomized, controlled trials in Brazil, the U.K., and South Africa. As per their interim analysis (published in December 2020), trial participants were in one of two cohorts: the standard-dose cohort (receiving two doses with 5x1010 viral particles each) and the low-dose cohort (one dose with 2.5x1010 viral particles, and one dose with 5x1010 viral particles). For all cases, the doses were administered 28 days apart. The vaccine efficacy (VE) in the standard-dose cohort was 63.1%, while the VE in the low-dose cohort was 80.7%, with an overall VE of 66.7%. However, more recent data according to a pre-print posted in the Lancet on February 2021 showed that by increasing the dose interval from less than six weeks to 12 weeks or more, the VE also increased from 54.9% to 82.4% in the standard-dose cohort, suggesting that VE, in Oxford-AstraZeneca’s case, increases with longer interval periods between the two doses.34 Further, as per another interim analysis report regarding the U.S. Phase 3 trials published in March , 2021, the Oxford-AstraZeneca vaccine was 76-79% effective at preventing symptomatic COVID-19, 100% effective at preventing severe disease and hospitalization, and 80-85% effective in participants aged 65 years and over.28,44 The U.S. participants received two standard doses, four weeks apart, following the promising data in the Lancet pre-print.28,44
ADDITIONAL INFORMATION REGARDING RESULTS OBSERVED IN PHASE 3 CLINICAL TRIALS
Oxford-AstraZeneca executed their blinded, multi-centre, randomized, controlled Phase 3 trials with over 17,000 individuals in the UK, Brazil, and South Africa.30 Oxford-AstraZeneca then measured the efficacy of their vaccine candidate 28 days after trial participants received their first dose and found that the overall vaccine efficacy was 66.7% 30,32 While trial participants in South Africa and Brazil received two standard doses (~5x1010 viral particles each), participants in the UK received either two standard doses (~5x1010 viral particles each), or one half dose (~2.5x1010 viral particles) and one standard dose.33 While collecting participants’ blood samples and conducting clinical assessments for information regarding the vaccine’s safety and immunogenicity, weekly swabbing was also done “for detection of infection and assessment of vaccine efficacy against infection.”30 For reasons that are yet to be determined, Oxford-AstraZeneca found that the vaccine efficacy was higher in the cohort of participants who received one half dose and one standard dose compared to the cohort of participants who received two standard doses only.16 Further analysis in a preprint under review in the Lancet reported that “a single standard dose of vaccine provided 76% protection overall against symptomatic COVID-19 in the first 90 days after vaccination with protection not falling in this time frame.”27,32 Also, with a dosing interval of 12 weeks or more, vaccine efficacy reached 82.4% after a second dose, compared to a 54.9% efficacy when the two doses were given less than six weeks apart.34,38 In the cohort of participants who received two standard doses, 22 hospitalization cases were reported in the placebo group compared to two cases in the vaccine group. No deaths were observed in the vaccine group, but one case of death was reported in the placebo group.34 Overall, 332 symptomatic cases were reported in the analysis population of over 17,000 participants.30 As of February 2021, Oxford-AstraZeneca have extended their study to assess the effects of their vaccine on children aged 6-17, with approximately 300 trial participants.39 On March 25, 2021, AstraZeneca published a news release, reporting the outcomes as per an interim analysis regarding the U.S. Phase 3 trials. In these trials, “trial participants aged 18 years or over who are healthy or have medically stable chronic diseases and are at increased risk for being exposed to the SARS-CoV-2 virus” received either two standard doses of the Oxford-AstraZeneca vaccine or saline placebos, four weeks apart.44 According to the news release, the Oxford-AstraZeneca vaccine was 76% effective at preventing symptomatic COVID-19, 100% effective against severe or critical disease and hospitalization, and 85% effective in participants aged 65 years and over.44 The vaccine efficacy was also consistent across ethnicity and age.44 Out of the 32,449 participants, 141 symptomatic cases were reported, with no increased risk of thrombosis or events characterized by thrombosis.44
More information about the Oxford-AstraZeneca vaccine and its Phase 3 trial can be found at:
- https://www.astrazeneca.com/content/astraz/media-centre/press-releases/2021/covid-19-vaccine-astrazeneca-confirms-protection-against-severe-disease-hospitalisation-and-death-in-the-primary-analysis-of-phase-iii-trials.html#
- https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)32661-1/fulltext
- https://www.astrazeneca.com/media-centre/press-releases/2021/astrazeneca-us-vaccine-trial-met-primary-endpoint.html
Janssen-Johnson & Johnson executed their blinded, randomized, controlled Phase 3 trials with over 43,000 individuals in the U.S., Latin America, and South Africa.17,40 Janssen-Johnson & Johnson then measured the efficacy of their vaccine candidate at two co-primary endpoints: 14 days post-vaccination, and 28 days post-vaccination.17 Trial participants were monitored daily and blood samples, saliva samples, and nasal swabs were collected.41 The efficacy of this vaccine varied geographically: 72% in the United States, 66% in Latin America, and 57% in South Africa.42 In all areas, there were no COVID-19 related hospitalization or death, 28 days post-vaccination.43 Further, the vaccine was 81.7% effective against severe forms of COVID-19 in South Africa, meaning that the Janssen-Johnson & Johnson vaccine can provide protection against serious disease caused by the South African SARS-CoV-2 variant.18 Overall, the vaccine was “85 percent effective in preventing severe disease across all regions studied.”43 468 symptomatic cases were reported after at least 14 days post-vaccination, and 261 symptomatic cases were reported at least 28 days post-vaccination in the analysis population of over 43,000 participants.18
More information about the Janssen-Johnson & Johnson vaccine and its Phase 3 trial can be found at:
- https://www.jnj.com/johnson-and-johnson-announces-single-shot-janssen-covid-19-vaccine-candidate-met-primary-endpoints-in-interim-analysis-of-its-phase-3-ensemble-trial
- https://www.jnj.com/coronavirus/ensemble-1-study-protocol
- https://www.jnj.com/coronavirus/about-phase-3-study-of-our-covid-19-vaccine-candidate
REFERENCES:
(1) CDC. Understanding Viral Vector COVID-19 Vaccines https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/viralvector.html (accessed Mar 17, 2021).
(2) Understanding and Explaining Viral Vector COVID-19 Vaccines | CDC https://www.cdc.gov/vaccines/covid-19/hcp/viral-vector-vaccine-basics.html (accessed Mar 17, 2021).
(3) What are viral vector-based vaccines and how could they be used against COVID-19? https://www.gavi.org/vaccineswork/what-are-viral-vector-based-vaccines-and-how-could-they-be-used-against-covid-19 (accessed Mar 17, 2021).
(4) University of Oxford. COVID-19 Oxford Vaccine Trial https://covid19vaccinetrial.co.uk/about (accessed Mar 17, 2021).
(5) Janssen Vaccines & Prevention B.V. A Randomized, Double-blind, Placebo-controlled Phase 3 Study to Assess the Efficacy and Safety of Ad26.COV2.S for the Prevention of SARS-CoV-2-mediated COVID-19 in Adults Aged 18 Years and Older https://www.jnj.com/coronavirus/ensemble-1-study-protocol (accessed Mar 17, 2021).
(6) Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural Basis for the Recognition of SARS-CoV-2 by Full-Length Human ACE2. Science 2020, 367 (6485), 1444–1448. https://doi.org/10.1126/science.abb2762.
(7) Cevik, M.; Kuppalli, K.; Kindrachuk, J.; Peiris, M. Virology, Transmission, and Pathogenesis of SARS-CoV-2. BMJ 2020, 371, m3862. https://doi.org/10.1136/bmj.m3862.
(8) The Central Dogma | Protocol https://www.jove.com/science-education/10798/the-central-dogma (accessed Mar 17, 2021).
(9) Explaining Johnson & Johnson’s, AstraZeneca’s new COVID-19 vaccines https://wexnermedical.osu.edu/blog/explaining-johnson-johnson-astrazeneca-vaccines (accessed Mar 17, 2021).
(10) Corum, J.; Zimmer, C. How the Johnson & Johnson Vaccine Works. The New York Times.
(11) Here’s Why Viral Vector Vaccines Don’t Alter DNA https://www.medpagetoday.com/special-reports/exclusives/91604 (accessed Mar 17, 2021).
(12) July 17, T. G.-A. M. E.; 2020. What are antibodies? https://www.livescience.com/antibodies.html (accessed Mar 17, 2021).
(13) Johnson, C.Y.; Steckelberg, A.; Sun, L.H.; McGinley, L. Your questions about coronavirus vaccines, answered https://washingtonpost.com/health/interactive/2020/covid-vaccines-what-you-need-to-know/ (accessed Mar 17, 2021).
(14) Zabner, J.; Couture, L. A.; Gregory, R. J.; Graham, S. M.; Smith, A. E.; Welsh, M. J. Adenovirus-Mediated Gene Transfer Transiently Corrects the Chloride Transport Defect in Nasal Epithelia of Patients with Cystic Fibrosis. Cell 1993, 75 (2), 207–216. https://doi.org/10.1016/0092-8674(93)80063-k.
(15) Ebola | Johnson & Johnson https://www.jnj.com/ebola (accessed Mar 17, 2021).
(16) AZD1222 vaccine met primary efficacy endpoint in preventing COVID-19 https://www.astrazeneca.com/media-centre/press-releases/2020/azd1222hlr.html (accessed Mar 17, 2021).
(17) Johnson & Johnson Announces Single-Shot Janssen COVID-19 Vaccine Candidate Met Primary Endpoints in Interim Analysis of its Phase 3 ENSEMBLE Trial | Johnson & Johnson https://www.jnj.com/johnson-and-johnson-announces-single-shot-janssen-covid-19-vaccine-candidate-met-primary-endpoints-in-interim-analysis-of-its-phase-3-ensemble-trial (accessed Mar 17, 2021).
(18) Janssen Biotech, Inc. VRBPAC Briefing Document - COVID-19 Vaccine Ad26.COV2.S https://www.fda.gov/media/146219/download (accessed Mar 17, 2021).
(19) Don’t Be Picky About Which Vaccine You Get Based On Efficacy Rates: Experts https://www.huffingtonpost.ca/entry/covid-vaccine-efficacy-rates_ca_6041897cc5b660a0f3874ca8 (accessed Mar 17, 2021).
(20) PINHO, A. C. COVID-19 Vaccine AstraZeneca: PRAC investigating cases of thromboembolic events - vaccine’s benefits currently still outweigh risks Update https://www.ema.europa.eu/en/news/covid-19-vaccine-astrazeneca-prac-investigating-cases-thromboembolic-events-vaccines-benefits (accessed Mar 17, 2021).
(21) Update on the safety of COVID-19 Vaccine AstraZeneca https://www.astrazeneca.com/media-centre/press-releases/2021/update-on-the-safety-of-covid-19-vaccine-astrazeneca.html (accessed Mar 17, 2021).
(22) Thrombosis Canada Statement on AstraZeneca COVID-19 Vaccine and Thrombosis. Thrombosis Canada - Thrombose Canada, 2021.
(23) PINHO, A. C. COVID-19 Vaccine AstraZeneca: benefits still outweigh the risks despite possible link to rare blood clots with low platelets https://www.ema.europa.eu/en/news/covid-19-vaccine-astrazeneca-benefits-still-outweigh-risks-despite-possible-link-rare-blood-clots (accessed Mar 19, 2021).
(24) Heit, J. A. Epidemiology of Venous Thromboembolism. Nat. Rev. Cardiol. 2015, 12 (8), 464–474. https://doi.org/10.1038/nrcardio.2015.83.
(25) CDC. Data and Statistics on Venous Thromboembolism | CDC https://www.cdc.gov/ncbddd/dvt/data.html (accessed Mar 19, 2021).
(26) WHO statement on AstraZeneca COVID-19 vaccine safety signals https://www.who.int/news/item/17-03-2021-who-statement-on-astrazeneca-covid-19-vaccine-safety-signals (accessed Mar 19, 2021).
(27) Malas, M. B.; Naazie, I. N.; Elsayed, N.; Mathlouthi, A.; Marmor, R.; Clary, B. Thromboembolism Risk of COVID-19 Is High and Associated with a Higher Risk of Mortality: A Systematic Review and Meta-Analysis. EClinicalMedicine 2020, 29. https://doi.org/10.1016/j.eclinm.2020.100639.
(28) AZD1222 US Phase III trial met primary efficacy endpoint in preventing COVID-19 at interim analysis https://www.astrazeneca.com/media-centre/press-releases/2021/astrazeneca-us-vaccine-trial-met-primary-endpoint.html (accessed Mar 22, 2021).
(29) Admin, O. W. COVID-19 mRNA Vaccines https://www.getwellclinic.ca/guides/vaccines/186-covid19-mrna-vaccine (accessed Mar 18, 2021).
(30) COVID-19 Vaccine AstraZeneca confirms 100% protection against severe disease, hospitalisation and death in the primary analysis of Phase III trials https://www.astrazeneca.com/media-centre/press-releases/2021/covid-19-vaccine-astrazeneca-confirms-protection-against-severe-disease-hospitalisation-and-death-in-the-primary-analysis-of-phase-iii-trials.html (accessed Mar 18, 2021).
(31) Vaccine Technology https://www.janssen.com/infectious-diseases-and-vaccines/vaccine-technology (accessed Mar 18, 2021).
(32) AstraZeneca’s COVID-19 vaccine authorised for emergency supply in the UK https://www.astrazeneca.com/media-centre/press-releases/2020/astrazenecas-covid-19-vaccine-authorised-in-uk.html (accessed Mar 18, 2021).
(33) Voysey, M.; Clemens, S. A. C.; Madhi, S. A.; Weckx, L. Y.; Folegatti, P. M.; Aley, P. K.; Angus, B.; Baillie, V. L.; Barnabas, S. L.; Bhorat, Q. E.; Bibi, S.; Briner, C.; Cicconi, P.; Collins, A. M.; Colin-Jones, R.; Cutland, C. L.; Darton, T. C.; Dheda, K.; Duncan, C. J. A.; Emary, K. R. W.; Ewer, K. J.; Fairlie, L.; Faust, S. N.; Feng, S.; Ferreira, D. M.; Finn, A.; Goodman, A. L.; Green, C. M.; Green, C. A.; Heath, P. T.; Hill, C.; Hill, H.; Hirsch, I.; Hodgson, S. H. C.; Izu, A.; Jackson, S.; Jenkin, D.; Joe, C. C. D.; Kerridge, S.; Koen, A.; Kwatra, G.; Lazarus, R.; Lawrie, A. M.; Lelliott, A.; Libri, V.; Lillie, P. J.; Mallory, R.; Mendes, A. V. A.; Milan, E. P.; Minassian, A. M.; McGregor, A.; Morrison, H.; Mujadidi, Y. F.; Nana, A.; O’Reilly, P. J.; Padayachee, S. D.; Pittella, A.; Plested, E.; Pollock, K. M.; Ramasamy, M. N.; Rhead, S.; Schwarzbold, A. V.; Singh, N.; Smith, A.; Song, R.; Snape, M. D.; Sprinz, E.; Sutherland, R. K.; Tarrant, R.; Thomson, E. C.; Török, M. E.; Toshner, M.; Turner, D. P. J.; Vekemans, J.; Villafana, T. L.; Watson, M. E. E.; Williams, C. J.; Douglas, A. D.; Hill, A. V. S.; Lambe, T.; Gilbert, S. C.; Pollard, A. J.; Aban, M.; Abayomi, F.; Abeyskera, K.; Aboagye, J.; Adam, M.; Adams, K.; Adamson, J.; Adelaja, Y. A.; Adewetan, G.; Adlou, S.; Ahmed, K.; Akhalwaya, Y.; Akhalwaya, S.; Alcock, A.; Ali, A.; Allen, E. R.; Allen, L.; Almeida, T. C. D. S. C.; Alves, M. P. S.; Amorim, F.; Andritsou, F.; Anslow, R.; Appleby, M.; Arbe-Barnes, E. H.; Ariaans, M. P.; Arns, B.; Arruda, L.; Azi, P.; Azi, L.; Babbage, G.; Bailey, C.; Baker, K. F.; Baker, M.; Baker, N.; Baker, P.; Baldwin, L.; Baleanu, I.; Bandeira, D.; Bara, A.; Barbosa, M. A. S.; Barker, D.; Barlow, G. D.; Barnes, E.; Barr, A. S.; Barrett, J. R.; Barrett, J.; Bates, L.; Batten, A.; Beadon, K.; Beales, E.; Beckley, R.; Belij-Rammerstorfer, S.; Bell, J.; Bellamy, D.; Bellei, N.; Belton, S.; Berg, A.; Bermejo, L.; Berrie, E.; Berry, L.; Berzenyi, D.; Beveridge, A.; Bewley, K. R.; Bexhell, H.; Bhikha, S.; Bhorat, A. E.; Bhorat, Z. E.; Bijker, E.; Birch, G.; Birch, S.; Bird, A.; Bird, O.; Bisnauthsing, K.; Bittaye, M.; Blackstone, K.; Blackwell, L.; Bletchly, H.; Blundell, C. L.; Blundell, S. R.; Bodalia, P.; Boettger, B. C.; Bolam, E.; Boland, E.; Bormans, D.; Borthwick, N.; Bowring, F.; Boyd, A.; Bradley, P.; Brenner, T.; Brown, P.; Brown, C.; Brown-O’Sullivan, C.; Bruce, S.; Brunt, E.; Buchan, R.; Budd, W.; Bulbulia, Y. A.; Bull, M.; Burbage, J.; Burhan, H.; Burn, A.; Buttigieg, K. R.; Byard, N.; Cabera Puig, I.; Calderon, G.; Calvert, A.; Camara, S.; Cao, M.; Cappuccini, F.; Cardoso, J. R.; Carr, M.; Carroll, M. W.; Carson-Stevens, A.; Carvalho, Y. de M.; Carvalho, J. A. M.; Casey, H. R.; Cashen, P.; Castro, T.; Castro, L. C.; Cathie, K.; Cavey, A.; Cerbino-Neto, J.; Chadwick, J.; Chapman, D.; Charlton, S.; Chelysheva, I.; Chester, O.; Chita, S.; Cho, J.-S.; Cifuentes, L.; Clark, E.; Clark, M.; Clarke, A.; Clutterbuck, E. A.; Collins, S. L. K.; Conlon, C. P.; Connarty, S.; Coombes, N.; Cooper, C.; Cooper, R.; Cornelissen, L.; Corrah, T.; Cosgrove, C.; Cox, T.; Crocker, W. E. M.; Crosbie, S.; Cullen, L.; Cullen, D.; Cunha, D. R. M. F.; Cunningham, C.; Cuthbertson, F. C.; Da Guarda, S. N. F.; da Silva, L. P.; Damratoski, B. E.; Danos, Z.; Dantas, M. T. D. C.; Darroch, P.; Datoo, M. S.; Datta, C.; Davids, M.; Davies, S. L.; Davies, H.; Davis, E.; Davis, J.; Davis, J.; De Nobrega, M. M. D.; De Oliveira Kalid, L. M.; Dearlove, D.; Demissie, T.; Desai, A.; Di Marco, S.; Di Maso, C.; Dinelli, M. I. S.; Dinesh, T.; Docksey, C.; Dold, C.; Dong, T.; Donnellan, F. R.; Dos Santos, T.; dos Santos, T. G.; Dos Santos, E. P.; Douglas, N.; Downing, C.; Drake, J.; Drake-Brockman, R.; Driver, K.; Drury, R.; Dunachie, S. J.; Durham, B. S.; Dutra, L.; Easom, N. J. W.; van Eck, S.; Edwards, M.; Edwards, N. J.; El Muhanna, O. M.; Elias, S. C.; Elmore, M.; English, M.; Esmail, A.; Essack, Y. M.; Farmer, E.; Farooq, M.; Farrar, M.; Farrugia, L.; Faulkner, B.; Fedosyuk, S.; Felle, S.; Feng, S.; Ferreira Da Silva, C.; Field, S.; Fisher, R.; Flaxman, A.; Fletcher, J.; Fofie, H.; Fok, H.; Ford, K. J.; Fowler, J.; Fraiman, P. H. A.; Francis, E.; Franco, M. M.; Frater, J.; Freire, M. S. M.; Fry, S. H.; Fudge, S.; Furze, J.; Fuskova, M.; Galian-Rubio, P.; Galiza, E.; Garlant, H.; Gavrila, M.; Geddes, A.; Gibbons, K. A.; Gilbride, C.; Gill, H.; Glynn, S.; Godwin, K.; Gokani, K.; Goldoni, U. C.; Goncalves, M.; Gonzalez, I. G. S.; Goodwin, J.; Goondiwala, A.; Gordon-Quayle, K.; Gorini, G.; Grab, J.; Gracie, L.; Greenland, M.; Greenwood, N.; Greffrath, J.; Groenewald, M. M.; Grossi, L.; Gupta, G.; Hackett, M.; Hallis, B.; Hamaluba, M.; Hamilton, E.; Hamlyn, J.; Hammersley, D.; Hanrath, A. T.; Hanumunthadu, B.; Harris, S. A.; Harris, C.; Harris, T.; Harrison, T. D.; Harrison, D.; Hart, T. C.; Hartnell, B.; Hassan, S.; Haughney, J.; Hawkins, S.; Hay, J.; Head, I.; Henry, J.; Hermosin Herrera, M.; Hettle, D. B.; Hill, J.; Hodges, G.; Horne, E.; Hou, M. M.; Houlihan, C.; Howe, E.; Howell, N.; Humphreys, J.; Humphries, H. E.; Hurley, K.; Huson, C.; Hyder-Wright, A.; Hyams, C.; Ikram, S.; Ishwarbhai, A.; Ivan, M.; Iveson, P.; Iyer, V.; Jackson, F.; De Jager, J.; Jaumdally, S.; Jeffers, H.; Jesudason, N.; Jones, B.; Jones, K.; Jones, E.; Jones, C.; Jorge, M. R.; Jose, A.; Joshi, A.; Júnior, E. A. M. S.; Kadziola, J.; Kailath, R.; Kana, F.; Karampatsas, K.; Kasanyinga, M.; Keen, J.; Kelly, E. J.; Kelly, D. M.; Kelly, D.; Kelly, S.; Kerr, D.; Kfouri, R. de Á.; Khan, L.; Khozoee, B.; Kidd, S.; Killen, A.; Kinch, J.; Kinch, P.; King, L. D. W.; King, T. B.; Kingham, L.; Klenerman, P.; Knapper, F.; Knight, J. C.; Knott, D.; Koleva, S.; Lang, M.; Lang, G.; Larkworthy, C. W.; Larwood, J. P. J.; Law, R.; Lazarus, E. M.; Leach, A.; Lees, E. A.; Lemm, N.-M.; Lessa, A.; Leung, S.; Li, Y.; Lias, A. M.; Liatsikos, K.; Linder, A.; Lipworth, S.; Liu, S.; Liu, X.; Lloyd, A.; Lloyd, S.; Loew, L.; Lopez Ramon, R.; Lora, L.; Lowthorpe, V.; Luz, K.; MacDonald, J. C.; MacGregor, G.; Madhavan, M.; Mainwaring, D. O.; Makambwa, E.; Makinson, R.; Malahleha, M.; Malamatsho, R.; Mallett, G.; Mansatta, K.; Maoko, T.; Mapetla, K.; Marchevsky, N. G.; Marinou, S.; Marlow, E.; Marques, G. N.; Marriott, P.; Marshall, R. P.; Marshall, J. L.; Martins, F. J.; Masenya, M.; Masilela, M.; Masters, S. K.; Mathew, M.; Matlebjane, H.; Matshidiso, K.; Mazur, O.; Mazzella, A.; McCaughan, H.; McEwan, J.; McGlashan, J.; McInroy, L.; McIntyre, Z.; McLenaghan, D.; McRobert, N.; McSwiggan, S.; Megson, C.; Mehdipour, S.; Meijs, W.; Mendonça, R. N. Á.; Mentzer, A. J.; Mirtorabi, N.; Mitton, C.; Mnyakeni, S.; Moghaddas, F.; Molapo, K.; Moloi, M.; Moore, M.; Moraes-Pinto, M. I.; Moran, M.; Morey, E.; Morgans, R.; Morris, S.; Morris, S.; Morris, H. C.; Morselli, F.; Morshead, G.; Morter, R.; Mottal, L.; Moultrie, A.; Moya, N.; Mpelembue, M.; Msomi, S.; Mugodi, Y.; Mukhopadhyay, E.; Muller, J.; Munro, A.; Munro, C.; Murphy, S.; Mweu, P.; Myasaki, C. H.; Naik, G.; Naker, K.; Nastouli, E.; Nazir, A.; Ndlovu, B.; Neffa, F.; Njenga, C.; Noal, H.; Noé, A.; Novaes, G.; Nugent, F. L.; Nunes, G.; O’Brien, K.; O’Connor, D.; Odam, M.; Oelofse, S.; Oguti, B.; Olchawski, V.; Oldfield, N. J.; Oliveira, M. G.; Oliveira, C.; Oosthuizen, A.; O’Reilly, P.; Osborne, P.; Owen, D. R. J.; Owen, L.; Owens, D.; Owino, N.; Pacurar, M.; Paiva, B. V. B.; Palhares, E. M. F.; Palmer, S.; Parkinson, S.; Parracho, H. M. R. T.; Parsons, K.; Patel, D.; Patel, B.; Patel, F.; Patel, K.; Patrick-Smith, M.; Payne, R. O.; Peng, Y.; Penn, E. J.; Pennington, A.; Peralta Alvarez, M. P.; Perring, J.; Perry, N.; Perumal, R.; Petkar, S.; Philip, T.; Phillips, D. J.; Phillips, J.; Phohu, M. K.; Pickup, L.; Pieterse, S.; Piper, J.; Pipini, D.; Plank, M.; Du Plessis, J.; Pollard, S.; Pooley, J.; Pooran, A.; Poulton, I.; Powers, C.; Presa, F. B.; Price, D. A.; Price, V.; Primeira, M.; Proud, P. C.; Provstgaard-Morys, S.; Pueschel, S.; Pulido, D.; Quaid, S.; Rabara, R.; Radford, A.; Radia, K.; Rajapaska, D.; Rajeswaran, T.; Ramos, A. S. F.; Ramos Lopez, F.; Rampling, T.; Rand, J.; Ratcliffe, H.; Rawlinson, T.; Rea, D.; Rees, B.; Reiné, J.; Resuello-Dauti, M.; Reyes Pabon, E.; Ribiero, C. M.; Ricamara, M.; Richter, A.; Ritchie, N.; Ritchie, A. J.; Robbins, A. J.; Roberts, H.; Robinson, R. E.; Robinson, H.; Rocchetti, T. T.; Rocha, B. P.; Roche, S.; Rollier, C.; Rose, L.; Ross Russell, A. L.; Rossouw, L.; Royal, S.; Rudiansyah, I.; Ruiz, S.; Saich, S.; Sala, C.; Sale, J.; Salman, A. M.; Salvador, N.; Salvador, S.; Sampaio, M.; Samson, A. D.; Sanchez-Gonzalez, A.; Sanders, H.; Sanders, K.; Santos, E.; Santos Guerra, M. F. S.; Satti, I.; Saunders, J. E.; Saunders, C.; Sayed, A.; Schim van der Loeff, I.; Schmid, A. B.; Schofield, E.; Screaton, G.; Seddiqi, S.; Segireddy, R. R.; Senger, R.; Serrano, S.; Shah, R.; Shaik, I.; Sharpe, H. E.; Sharrocks, K.; Shaw, R.; Shea, A.; Shepherd, A.; Shepherd, J. G.; Shiham, F.; Sidhom, E.; Silk, S. E.; da Silva Moraes, A. C.; Silva-Junior, G.; Silva-Reyes, L.; Silveira, A. D.; Silveira, M. B. V.; Sinha, J.; Skelly, D. T.; Smith, D. C.; Smith, N.; Smith, H. E.; Smith, D. J.; Smith, C. C.; Soares, A.; Soares, T.; Solórzano, C.; Sorio, G. L.; Sorley, K.; Sosa-Rodriguez, T.; Souza, C. M. C. D. L.; Souza, B. S. D. F.; Souza, A. R.; Spencer, A. J.; Spina, F.; Spoors, L.; Stafford, L.; Stamford, I.; Starinskij, I.; Stein, R.; Steven, J.; Stockdale, L.; Stockwell, L. V.; Strickland, L. H.; Stuart, A. C.; Sturdy, A.; Sutton, N.; Szigeti, A.; Tahiri-Alaoui, A.; Tanner, R.; Taoushanis, C.; Tarr, A. W.; Taylor, K.; Taylor, U.; Taylor, I. J.; Taylor, J.; te Water Naude, R.; Themistocleous, Y.; Themistocleous, A.; Thomas, M.; Thomas, K.; Thomas, T. M.; Thombrayil, A.; Thompson, F.; Thompson, A.; Thompson, K.; Thompson, A.; Thomson, J.; Thornton-Jones, V.; Tighe, P. J.; Tinoco, L. A.; Tiongson, G.; Tladinyane, B.; Tomasicchio, M.; Tomic, A.; Tonks, S.; Towner, J.; Tran, N.; Tree, J.; Trillana, G.; Trinham, C.; Trivett, R.; Truby, A.; Tsheko, B. L.; Turabi, A.; Turner, R.; Turner, C.; Ulaszewska, M.; Underwood, B. R.; Varughese, R.; Verbart, D.; Verheul, M.; Vichos, I.; Vieira, T.; Waddington, C. S.; Walker, L.; Wallis, E.; Wand, M.; Warbick, D.; Wardell, T.; Warimwe, G.; Warren, S. C.; Watkins, B.; Watson, E.; Webb, S.; Webb-Bridges, A.; Webster, A.; Welch, J.; Wells, J.; West, A.; White, C.; White, R.; Williams, P.; Williams, R. L.; Winslow, R.; Woodyer, M.; Worth, A. T.; Wright, D.; Wroblewska, M.; Yao, A.; Zimmer, R.; Zizi, D.; Zuidewind, P. Safety and Efficacy of the ChAdOx1 NCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. The Lancet 2021, 397 (10269), 99–111. https://doi.org/10.1016/S0140-6736(20)32661-1.
(34) Voysey, M.; Costa Clemens, S. A.; Madhi, S. A.; Weckx, L. Y.; Folegatti, P. M.; Aley, P. K.; Angus, B. J.; Baillie, V.; Barnabas, S. L.; Bhorat, Q. E.; Bibi, S.; Briner, C.; Cicconi, P.; Clutterbuck, E.; Collins, A. M.; Cutland, C.; Darton, T.; Dheda, K.; Douglas, A. D.; Duncan, C. J. A.; Emary, K. R. W.; Ewer, K.; Flaxman, A.; Fairlie, L.; Faust, S. N.; Feng, S.; Ferreira, D. M.; Finn, A.; Galiza, E.; Goodman, A. L.; Green, C. M.; Green, C. A.; Greenland, M.; Hill, C.; Hill, H. C.; Hirsch, I.; Izu, A.; Jenkin, D.; Kerridge, S.; Koen, A.; Kwatra, G.; Lazarus, R.; Libri, V.; Lillie, P. J.; Marchevsky, N. G.; Marshall, R. P.; Mendes, A. V. A.; Milan, E. P.; Minassian, A. M.; McGregor, A. C.; Farooq Mujadidi, Y.; Nana, A.; Payadachee, S. D.; Phillips, D. J.; Pittella, A.; Plested, E.; Pollock, K. M.; Ramasamy, M. N.; Robinson, H.; Schwarzbold, A. V.; Smith, A.; Song, R.; Snape, M. D.; Sprinz, E.; Sutherland, R. K.; Thomson, E. C.; Torok, M.; Toshner, M.; Turner, D. P. J.; Vekemans, J.; Villafana, T. L.; White, T.; Williams, C. J.; Hill, A. V. S.; Lambe, T.; Gilbert, S. C.; Pollard, A.; Group, O. C. V. T. Single Dose Administration, And The Influence Of The Timing Of The Booster Dose On Immunogenicity and Efficacy Of ChAdOx1 NCoV-19 (AZD1222) Vaccine; SSRN Scholarly Paper ID 3777268; Social Science Research Network: Rochester, NY, 2021. https://doi.org/10.2139/ssrn.3777268.
(35) Information for UK recipients on COVID 19 Vaccine AstraZeneca https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-astrazeneca/information-for-uk-recipients-on-covid-19-vaccine-astrazeneca (accessed Mar 18, 2021).
(36) CDC. Information about Johnson & Johnson's Janssen COVID-19 Vaccine https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/janssen.html (accessed Mar 18, 2021).
(37) Phase 3 Clinical Testing in the US of AstraZeneca COVID-19 Vaccine Candidate Begins https://www.nih.gov/news-events/news-releases/phase-3-clinical-testing-us-astrazeneca-covid-19-vaccine-candidate-begins (accessed Mar 18, 2021).
(38) Wise, J. Covid-19: New Data on Oxford AstraZeneca Vaccine Backs 12 Week Dosing Interval. BMJ 2021, 372, n326. https://doi.org/10.1136/bmj.n326.
(39) Oxford coronavirus vaccine childrens study - FAQs https://www.research.ox.ac.uk/Article/2021-02-15-oxford-coronavirus-vaccine-childrens-study-faqs (accessed Mar 18, 2021).
(40) Johnson & Johnson Initiates Pivotal Global Phase 3 Clinical Trial of Janssen’s COVID-19 Vaccine Candidate | Johnson & Johnson https://www.jnj.com/johnson-johnson-initiates-pivotal-global-phase-3-clinical-trial-of-janssens-covid-19-vaccine-candidate (accessed Mar 18, 2021).
(41) About Our ENSEMBLE Studies https://www.jnj.com/coronavirus/about-phase-3-study-of-our-covid-19-vaccine-candidate (accessed Mar 18, 2021).
(42) Janssen Investigational COVID-19 Vaccine: Interim Analysis of Phase 3 Clinical Data Released https://www.nih.gov/news-events/news-releases/janssen-investigational-covid-19-vaccine-interim-analysis-phase-3-clinical-data-released (accessed Mar 18, 2021).
(43) Johnson & Johnson COVID-19 Vaccine Authorized by U.S. FDA For Emergency Use | Johnson & Johnson https://www.jnj.com/johnson-johnson-covid-19-vaccine-authorized-by-u-s-fda-for-emergency-usefirst-single-shot-vaccine-in-fight-against-global-pandemic (accessed Mar 17, 2021).
(44) AZD1222 US Phase III primary analysis confirms safety and efficacy , https://www.astrazeneca.com/media-centre/press-releases/2021/azd1222-us-phase-iii-primary-analysis-confirms-safety-and-efficacy.html (accessed Mar 25, 2021).